Controlled Substance Inventory Log free printable template
Show details
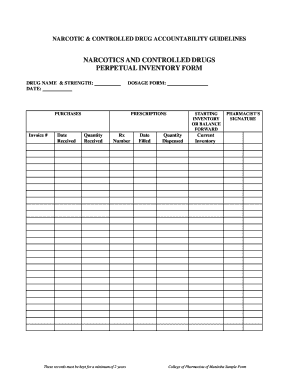
SIGNATURE. DATE. RECEIVED. INVOICE×. QUANTITY. RECEIVED. DEFILED PRESCRIPTION×. QUANTITY. DISPENSED. CURRENT. INVENTORY. This is a sample form for use by pharmacies using a pharmacy system that
pdfFiller is not affiliated with any government organization
Get, Create, Make and Sign printable narcotic log sheet form

Edit your printable controlled substance log form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your controlled substance log form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing controlled substance log sheet online
To use our professional PDF editor, follow these steps:
1
Check your account. It's time to start your free trial.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit printable controlled drug log sheets form. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out controlled substance inventory log form

How to fill out Controlled Substance Inventory Log
01
Gather all necessary information about the controlled substances to be logged.
02
Obtain a blank Controlled Substance Inventory Log template or use a digital logging system.
03
Start with the date and time of the inventory.
04
Record the name of the substance, including its strength and form (e.g., tablet, liquid).
05
Note the quantity on hand for each controlled substance.
06
Enter the location where the controlled substance is stored.
07
Include the name of the person conducting the inventory.
08
Sign and date the log to verify accuracy.
09
Make sure to keep the log in a secure location and maintain confidentiality.
Who needs Controlled Substance Inventory Log?
01
Healthcare professionals who handle controlled substances, including pharmacists, nurses, and doctors.
02
Facilities such as hospitals, clinics, and pharmacies that store or administer controlled substances.
03
Regulatory agencies to ensure compliance with legal requirements for controlled substance handling.
Fill
controlled substance inventory log template
: Try Risk Free






People Also Ask about dea controlled substance log template
Which information is recorded in a controlled drug logbook?
Besides the “bound” requirement, the logbook must be maintained by the DEA registrant and include at a minimum all of the following information: drug name, container size, strength of medication, bottle number, date of dispensation, explanation of use, lot number (if available), expiration date, amount added to logbook
What is narcotic log book?
The Narcotic Book provides a tamper-proof record system for your facility. Enter each individual patient's name in the index at the front of the book, then record every drug administered to that patient on the corresponding consecutively numbered page in the book.
Which DEA form must be used to document the destruction of a controlled substance?
Registrant Record of Controlled Substances Destroyed - DEA Form 41.
What is DEA form 22 used for?
The Drug Enforcement Administration (DEA), Office of Diversion Control, will accept requests from distributors that require a large volume of Order Forms (DEA Form 222) with the pin feed tracking left on the form. Order Forms are used for the distribution of a Schedule I or II controlled substance.
What is the purpose of DEA form 41?
Additionally, a DEA Form 41 must be used when destroying expired or unwanted controlled substances. So, it's imperative to select a government-approved reverse distributor for the destruction of expired or unwanted controlled substances.
What must the inventory of controlled substances include?
Actual Name of Controlled Substance, Form, Quantity, Strength; ▪ Number of Units or Volume of Finished Form Dispensed; ▪ Name, Address of the Person to Whom It Was Dispensed; ▪ Date of Dispensing.
Our user reviews speak for themselves
Read more or give pdfFiller a try to experience the benefits for yourself
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my controlled substance log printable in Gmail?
controlled drug log sheets printable and other documents can be changed, filled out, and signed right in your Gmail inbox. You can use pdfFiller's add-on to do this, as well as other things. When you go to Google Workspace, you can find pdfFiller for Gmail. You should use the time you spend dealing with your documents and eSignatures for more important things, like going to the gym or going to the dentist.
How can I send pdffiller for eSignature?
When you're ready to share your controlled substance printable log, you can send it to other people and get the eSigned document back just as quickly. Share your PDF by email, fax, text message, or USPS mail. You can also notarize your PDF on the web. You don't have to leave your account to do this.
How do I execute controlled substance log template online?
Filling out and eSigning substance printable log controlled is now simple. The solution allows you to change and reorganize PDF text, add fillable fields, and eSign the document. Start a free trial of pdfFiller, the best document editing solution.
What is Controlled Substance Inventory Log?
A Controlled Substance Inventory Log is a record used by healthcare providers, pharmacies, and facilities to document the quantities of controlled substances they possess, including details about the receipt, distribution, and disposal of these substances.
Who is required to file Controlled Substance Inventory Log?
Pharmacies, healthcare facilities, and practitioners who handle controlled substances are required to file a Controlled Substance Inventory Log.
How to fill out Controlled Substance Inventory Log?
To fill out a Controlled Substance Inventory Log, one must record the name of the controlled substance, its dosage form, the quantity on hand, the date and time of the inventory, and the signature of the person conducting the inventory.
What is the purpose of Controlled Substance Inventory Log?
The purpose of the Controlled Substance Inventory Log is to ensure accountability, track the use and movement of controlled substances, and comply with legal and regulatory requirements.
What information must be reported on Controlled Substance Inventory Log?
Information that must be reported includes the name of the controlled substance, dosage form, strength, quantity on hand, date of inventory, location, and the signatures of those conducting and verifying the inventory.
Fill out your Controlled Substance Inventory Log online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Substance Printable Controlled Log is not the form you're looking for?Search for another form here.
Keywords relevant to printable controlled drug log
Related to controlled drug record sheet
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.